Explore our network of country and industry based websites to access localized information, product offerings, and business services across our group.
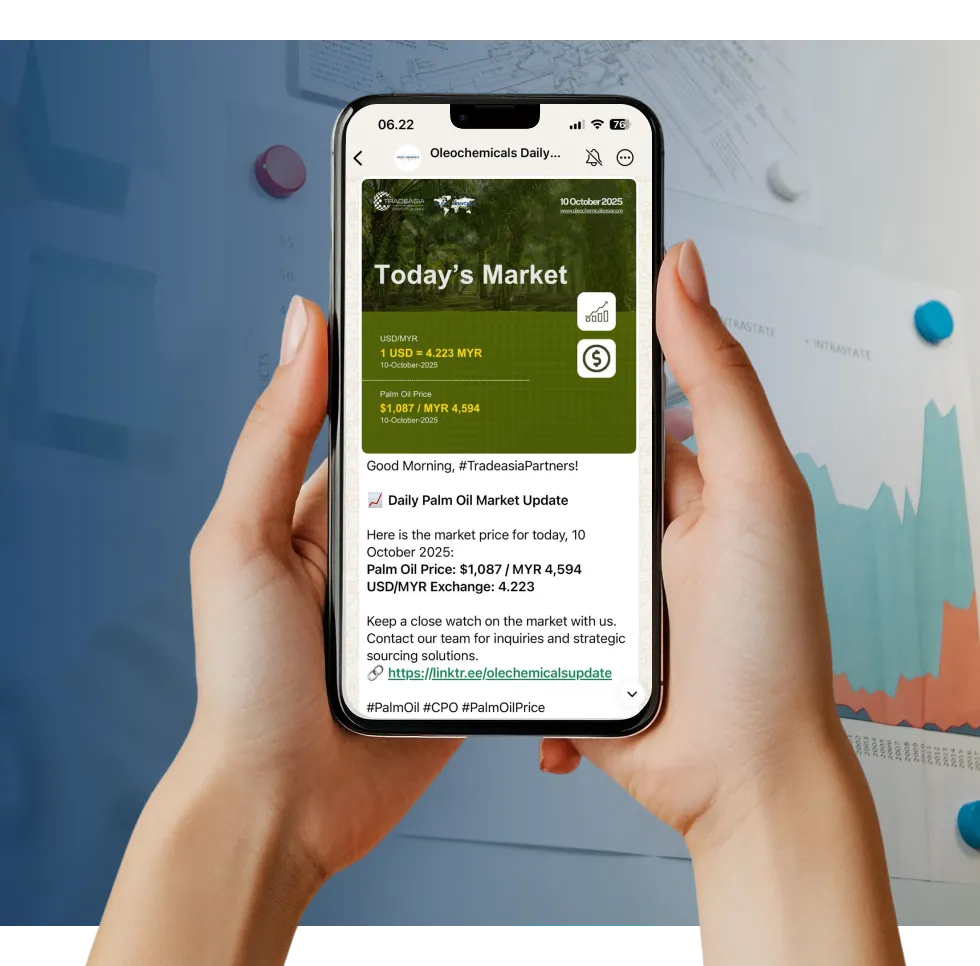
Access reliable chemical market information through our update channels.
Real-time Updates
Daily Updates
Log in to start sending quotation requests for any product.
Don't have an account? Sign Up Here
Log in now to access technical product documents from our product range.
Don't have an account? Sign Up Here
Home All Products Sodium Laureth Sulphate
|
IUPAC Name |
: sodium;2-[2-(2-dodecoxyethoxy)ethoxy]ethyl sulfate |
|
Cas Number |
: 13150-00-0 |
|
HS Code |
: 3402.11.10 |
|
Formula |
: CH3(CH2)11(OCH2CH2)nOSO3Na |
|
Appearance Name |
: White to Yellowish Powder or Needle |
|
Common Names |
: SLES, Sodium lauryl ether sulfate Sodium laureth sulphate Sodium lauryl ether sulphate |
|
Packaging |
: 20kg/bag, 10.5mt/fcl (N.W.),10.763mt/fcl (G.W.) without pallet |
For more detailed information including pricing, customization, and shipping:
Sodium laureth sulfate (SLES), an accepted contraction of sodium lauryl ether sulfate (SLES), is an anionic detergent and surfactant found in many personal care products (soaps, shampoos, toothpaste etc.). SLES is an inexpensive and very effective foaming agent. SLES, sodium lauryl sulfate (SLS), ammonium lauryl sulfate (ALS), and sodium pareth sulfate are surfactants that are used in many cosmetic products for their cleaning and emulsifying properties. They behave similarly to soap. Its chemical formula is CH3(CH2)11(OCH2CH2)nOSO3Na. Sometimes the number represented by n is specified in the name, for example laureth-2 sulfate. The product is heterogeneous in the number of ethoxyl groups, where n is the mean. It is common for commercial products for n= 3.
Sodium Laureth Sulfate (SLES) is a widely used surfactant in the production of personal care and cleaning products. The manufacturing process of SLES involves ethoxylation of lauryl alcohol followed by sulfation. Initially, lauryl alcohol undergoes ethoxylation, a process in which ethylene oxide is added to the alcohol molecule. This results in the formation of ethoxylated lauryl alcohol. Subsequently, the ethoxylated lauryl alcohol is sulfated by reacting it with sulfur trioxide or chlorosulfonic acid. Neutralization with sodium hydroxide follows, resulting in the formation of Sodium Laureth Sulfate. The final product is a versatile anionic surfactant known for its foaming and cleansing properties, making it a key ingredient in various personal care and cleaning formulations. However, it's essential to note that the production and use of SLES have raised environmental and health concerns, leading to increased efforts to explore alternative, more sustainable surfactants in the industry.
SLS is a highly effective anionic surfactant used to remove oily stains and residues. It is found in high concentrations in industrial products, including engine degreasers, floor cleaners, and car wash products, where workplace protections can be implemented to avoid unsafe exposures. SLS is also used in lower concentrations in household and personal care products such as cleaning products, toothpastes, shampoos, and shaving foams.

